Aptar Pharma Bolsters Clinical Trial Capabilities with Strategic Materials Manufacturing Acquisition

The acquisition of Mod3 Pharma’s clinical trial materials manufacturing capabilities strengthens Aptar Pharma’s service portfolio to meet growing demand for early-phase clinical trial support and innovation.
Driving Innovation in Inhaled and Nasal Drug Product Development

Explore how Nanopharm, a globally recognized Contract Research Organization (CRO), is becoming the inevitable choice for inhalation and nasal drug product development. Under the leadership of Gemma Budd, the new General Manager, Nanopharm has established itself as a Centre of Excellence in Orally Inhaled and Nasal Drug Products (OINDP). With its state-of-the-art, purpose-built facility, Nanopharm offers comprehensive inhalation services, characterized by an in-depth understanding of OINDP science, products, and test methods.
Nanopharm Announces Exclusive Collaboration With Fluidda To Accelerate Regulatory Pathway For OINDP Using SmartTrack

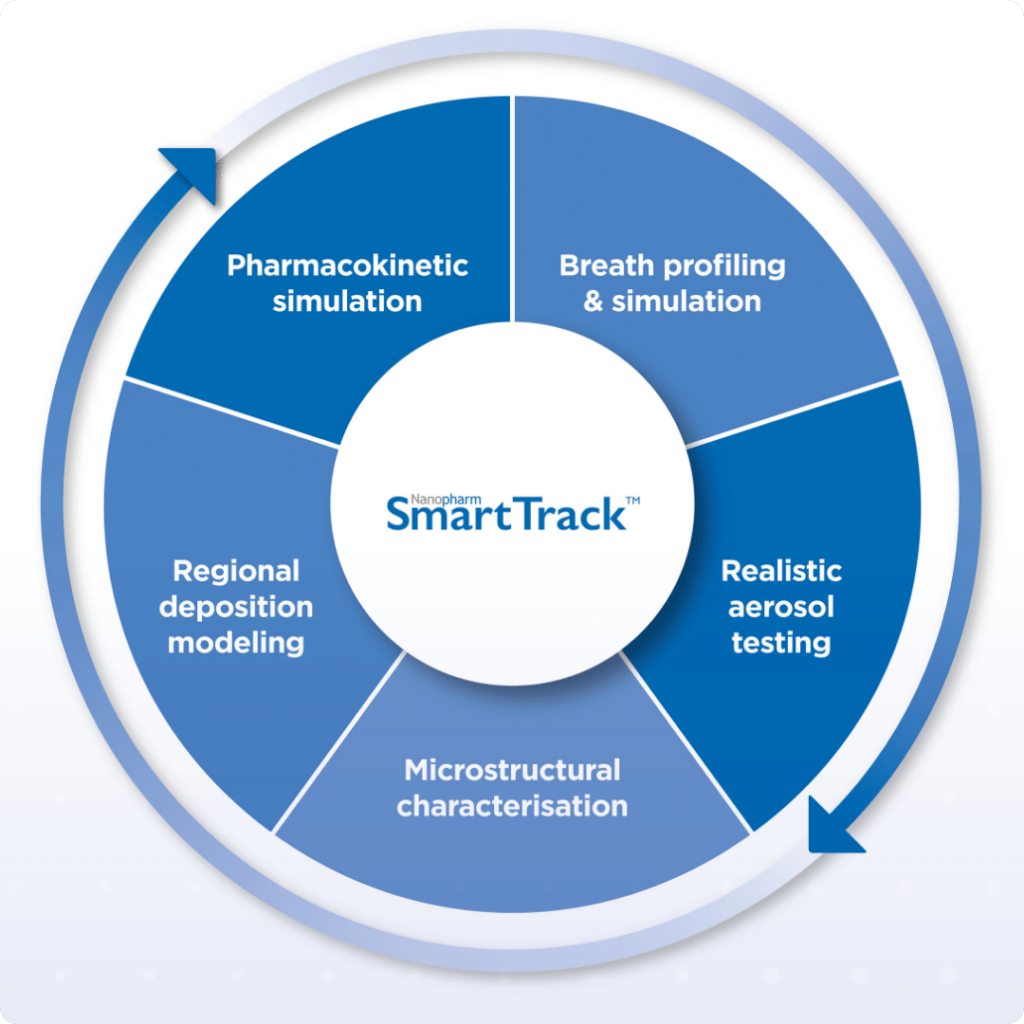
Crystal Lake, Illinois, September 22, 2022 – Nanopharm, an Aptar Pharma company and leader in contract research and development services for orally inhaled and nasal drug products (OINDPs), today announced an exclusive collaboration with Fluidda, a leader in the field of Functional Respiratory Imaging. The companies will leverage their respective proprietary technology platforms to help accelerate U.S. Food & Drug Administration (FDA) approvals for orally inhaled generic products (OIDPs) via the alternative bioequivalence pathway. Nanopharm was acquired by Aptar (NYSE: ATR) in 2019, as part of the company’s strategy to expand its services offerings and partner with pharmaceutical companies earlier in the drug development process.
Nanopharm & FLUIDDA Interviews on SmartTrack: In Vitro-In Silico Approaches for Demonstrating Bioequivalence for OINDP

To support generic drug companies in obtaining a market license for their orally inhaled drug products (OIDPs), Nanopharm and FLUIDDA entered into an exclusive collaboration to combine Nanopharm’s SmartTrack™ with FLUIDDA’s Functional Respiratory Imaging technology to create a unique in-vitro in-silico platform. This allows pharma companies to shorten their clinical pathways – and potentially avoid […]
A Holistic Demonstration of SmartTrack™ and New Ways of Collaborating Combine for a Successful FDA Workshop
Nasal spray innovation with micellar delivery of Cyclosporine A holds promise in combating SARS-CoV-2 infections
