News & Events
Nanopharm News & Events
Nanopharm is continually expanding its specialized nasal and inhaled drug delivery development capabilities and participates in numerous events within the OINDP pharmaceutical community. Learn more about the latest news and events at Nanopharm.
06 December 2024
Nanopharm Newsletter November 2024
This latest newsletter provides an overview of recent activities at Nanopharm as well as an interview with Dr Shaban, Head of Pharmaceutical Development.
Nanopharm, Newsletter, OINDPs

19 Apr 2024
Innovation & Insights

19 Apr 2024
Innovation & Insights
17 Apr 2024
Innovation & Insights
01 Jun 2023
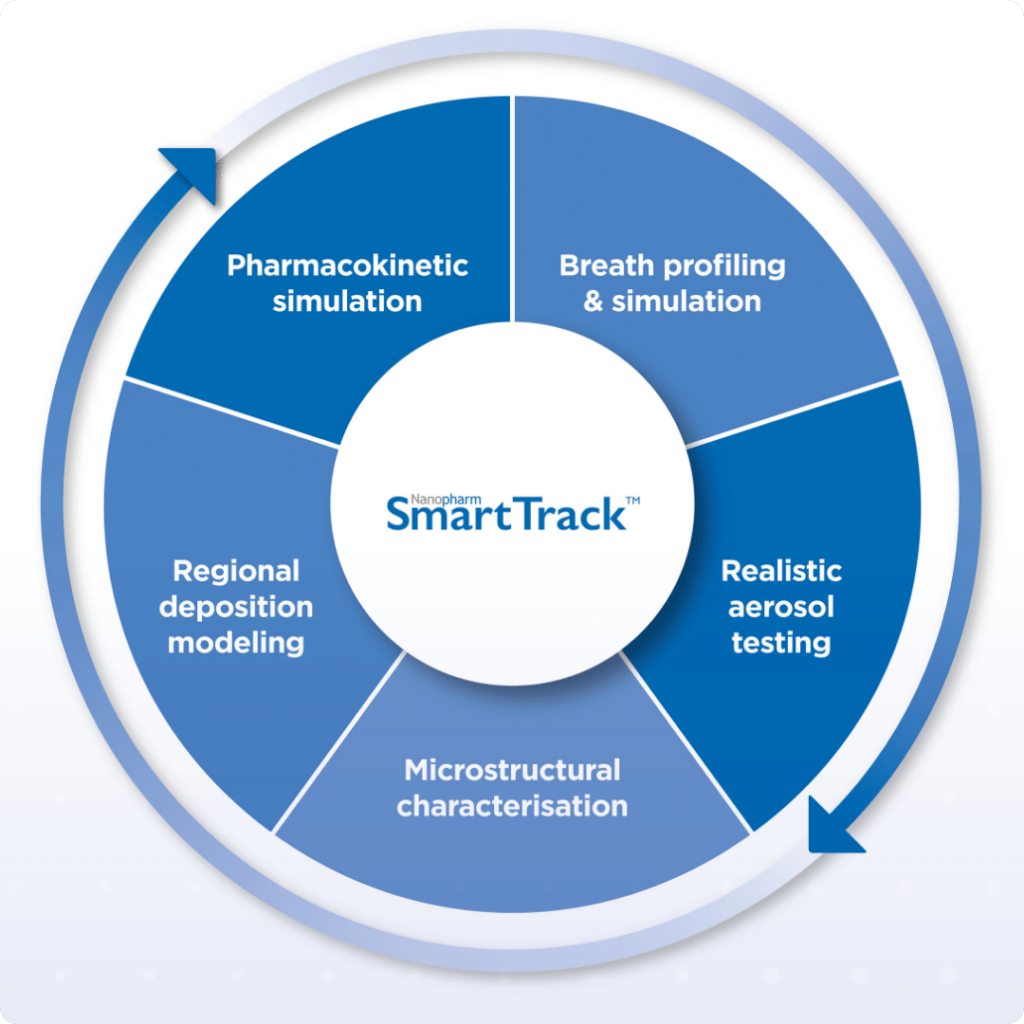
bioequivalence, Nanopharm, OINDP, PBPK modelling, Pharmacokinetic, SmartTrack
01 Dec 2022
Drug Product, Generic, Inhalation, OINDP, Pharmaceutical regulatory

01 Oct 2022
Aptar Pharma, OINDP, PBPK, PBPK models
11 May 2022
Biologics, COVID-19, Nasal, Nasal Delivery, therapeutics

15 Apr 2022
drug formulation, formulation and development, Nasal
14 Apr 2022
Biologics, drug delivery, Drug Development, Nanopharm, Nasal, SARS-CoV-2
06 Apr 2022
environmental footprint, Global Warming Potential, MDIs, metered dose inhalers, SmartTrack

