OINDP In-Silico Modeling
OINDP In-Silico Modeling Services
As specialists in the development of intranasal and inhaled drug products, Nanopharm has developed a range of in-silico based technologies that can accelerate and derisk the development of OINDPs. This unique scientific service offering comes from a strategic drive to make improved inhaled and/or nasal drug products faster with less time-consuming and lower cost clinical studies. We work closely with regulators to provide our customers with development pathways that provide these services with confidence. From regional deposition Modeling to Simhalation™ PBPK simulations and our proprietary SmartTrack™ platform for inhaled drug development, Nanopharm is a market leader in applying advanced in-silico models to improve OINDP development.
SmartTrack is the proprietary in-vitro in-silico Modeling platform that delivers an alternative and non-clinical pathway to bioequivalence for inhaled drug products.
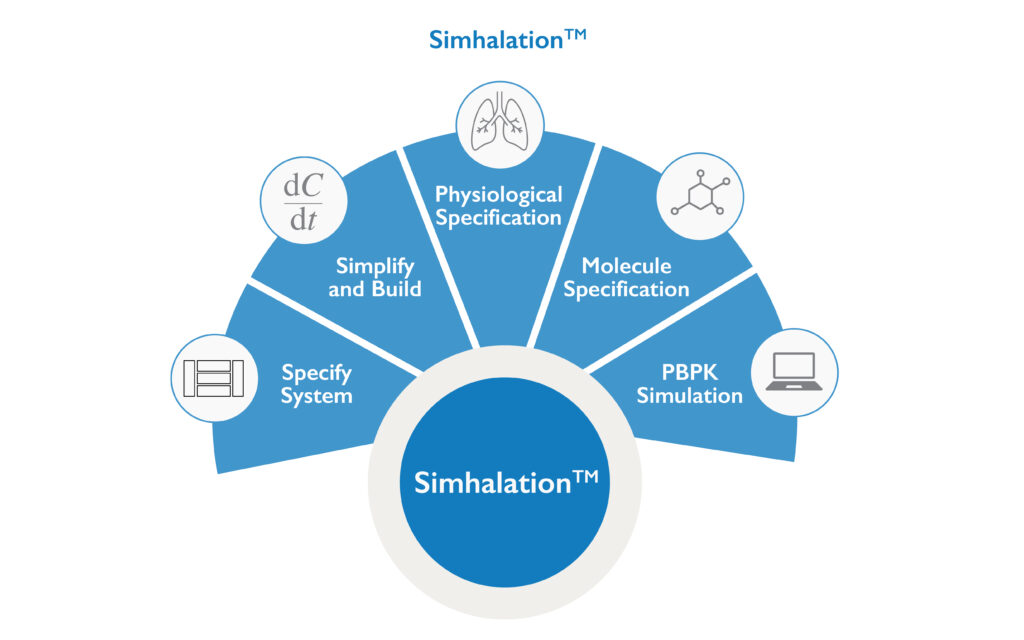
Nanopharm’s proprietary Physiologically-Based Pharmacokinetic (PBPK) platform, Simhalation™, models a drug’s transportation throughout the body to reliably predict each drug’s specific local and systemic PK profile in-silico, saving time, money and derisking clinical studies.
Nanopharm’s in-silico regional deposition models use computational fluid dynamics (CFD) to provide a critical understanding of drug deposition in the nose or lungs of healthy or diseased patients for accelerated OINDP optimization.
Nanopharm’s proprietary SmartTrack™ in-vitro in-silico Modeling platform provides an alternative non-clinical pathway to bioequivalence for inhaled drug products. This unique approach can eliminate the need for costly and time consuming comparative clinical endpoint (CCEP) studies through the application of a combination of our specialized technologies including breath profiling to regional deposition Modeling and pharmacokinetic simulations. SmartTrack™ is estimated to be able to save a company $30-$40 million dollars by eliminating the need for the CCEP study, and years of development time as a result. Developed in close cooperation with the U.S. FDA, SmartTrack™ represents an integrated assessment of the many and complex interactions between the API, formulation, device as well as the inherent patient variability using leading edge advanced in-vitro in-silico technologies. And you’ll only find SmartTrack™ at Nanopharm. Your experts in OINDP product development services.
Nanopharm’s Physiologically-Based Pharmacokinetic (PBPK) model for OINDPs is called Simhalation™. It uses unique mathematical descriptions of a drug’s transportation throughout the body describing everything from administration to excretion. This proprietary in-silico model allows Nanopharm’s OINDP scientists to reliably predict the drug’s pharmacokinetics (PKs) locally in the lungs or nose, as well as systemically, to screen and optimize dosing regimens, excipients or formulation enhancers.
Generating a strong understanding of each drug’s ADME (absorption, distribution, metabolism, and excretion) profile allows our scientists to clearly understand the drug’s rate and extent of release as well as its bioavailability at the site of action, all before going into clinical studies. Nanopharm’s PBPK Modeling derisks your PK studies and accelerates nasal or inhaled drug product development.
Through our collaboration with Fluidda and their FRI™ platform, Nanopharm uses a range of in-vivo and in-vitro data to populate its unique in-silico regional deposition models that can reliably predict the quantity of drug deposited in each region of the patient’s lung or nose, considering different disease states. Both intranasal and inhaled drugs present complex drug delivery pathways, so having a strong understanding of where the drug is actually going within the body can provide critical information that can ultimately influence overall drug delivery. Our scientists use this data to guide inhaled or nasal product formulation development, perform device selection and address human factor variability in the drug development process. Using all this data in advanced computational fluid-dynamics (CFD) Modeling allows Nanopharm’s scientists to develop highly representative regional deposition models for today’s complex OINDP products. This means we can accelerate and derisk OINDP product development using techniques that have been validated through SPECT CT and gamma scintigraphy studies.
Nanopharm Applies Leading Edge In-Silico Modeling for Better OINDP Development
Nanopharm applies a variety of highly specialized in-silico development tools designed specifically for intranasal and inhaled drug products. That combined with decades of experience in bringing customer OINDPs to market, Nanopharm is a recognized innovator in using the most advanced development Modeling techniques available. And the end result is, shorter development timelines, reduced risk and more robust products for our customers.