Services
For several years, Nanopharm has been a specialist Contract Research Organization (CRO) for nasal and inhaled drug products. By offering advanced analytical and development services, Nanopharm contributes to the acceleration and derisking of the development of nasal and inhaled drug products. We understand the direct relationship between drug product performance and clinical performance in patients. In fact, we take a holistic approach to OINDP drug development assessing critical factors including the material, the drug delivery system, and chemical attributes through a series of proprietary and leading-edge analytical tests, in-vitro and in-silico models as well as pharmacokinetic simulations, to arrive at realizable OINDP products. Our service offering is as extensive as it is focused, providing our customers with the support they need to advance their candidate molecules to market with less risk.
Nanopharm Specialist Nasal and Inhaled Drug Product Development Services
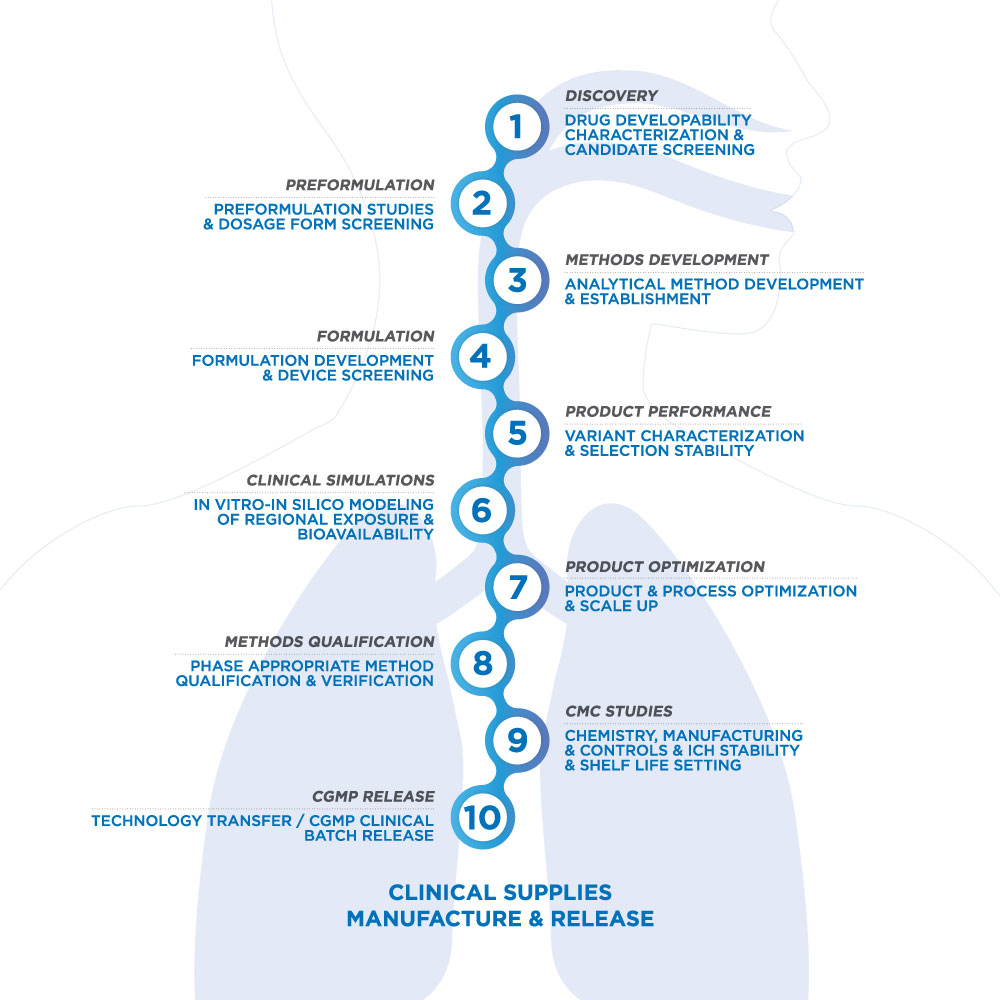
Nanopharm considers the complete product, from the discovery stage all the way through the development process, to better understand the interconnectivity between the device, the API, the formulation, and how a patient will use it.
Nanopharm has built a full-service analytical development and cGMP testing capability to support projects at every stage of their development.
Nanopharm offers a range of in-silico modeling tools and simulations that can predict a drug’s pharmacokinetic profile and the regional deposition of the delivered dose, for bridging studies or early pre-clinical studies.
Nanopharm’s team of regulatory experts offer specialized regulatory support for drug device combination products and their complex requirements.
OINDP product development is a complex process, requiring specialized technologies and knowledge in order to produce scalable and effective inhaled products. Nanopharm takes a holistic approach, from discovery all the way through the development process, to better understand the interconnectivity between the drug delivery technology, the API, the formulation, and the patient. With our unique in-vitro in-silico modeling platform (SmartTrack™) incorporating breathing profile simulations, anatomical mouth-throat geometries, patient and disease-specific lung geometries, and drug deposition studies, we can optimize formulations for each drug delivery system and application. Our product development programs include looking at a variety of manufacturing and filling processes, selection of excipients to improve functional performance, as well as potential drug formulation interactions with the primary packaging system. We bring a wide range of OINDP development tools and techniques together with our most experienced scientists to deliver fully optimized orally inhaled and intranasal drug product development services to each and every customer project.
Nanopharm has built a full-service analytical development and cGMP testing capability to support OINDP projects at every stage of development. Our analytical laboratories include specialized and proprietary testing capabilities created to support the added complexities of inhaled and nasal spray drug products. These include our Dissohale™ aerosol lung dose collection apparatus, immersion cells for dissolution and in-vitro release testing (IVRT), Nanopharm Inhalation Profiler (NIP) breath inhalation profiler, and Phase Doppler Anemometry (PDA). Our analytical laboratory covers a range of methods used for OINDP development and testing such as spray pattern/plume geometry, delivered dose uniformity, emitted droplet/particle size distribution, dissolution, IVRT, aerodynamic particle size distribution (APSD) and much more. Nanopharm also operates modern conventional analytical apparatus such as high-performance liquid chromatography (HPLC/uHPLC) with a range of detectors including mass spec (MS), UV-DAD, and much more. We can develop and validate the analytical test methods you need for your OINDP product. Our cGMP laboratory can also support testing and release for clinical or commercial batches.
Analysis of inhaled or nasal spray products is complex and requires a multidimensional approach. Studying the influence of individual patients on factors that impact how much drug reaches the target location and can be absorbed into the body are critical to developing an OINDP product. Nanopharm offers a range of in-silico modeling tools and simulations that can predict the drug’s pharmacokinetic profile and regional delivered dose, to derisk bridging studies or early pre-clinical studies. For example, our computational in-silico pharmaceutics platform can analyze regional deposition of the drug in the lung or nose through computational fluid dynamics (CFD) modeling. Our unique approach to in-silico physiological based pharmacokinetic modeling (PBPK) studies assesses many parameters including blood flow and tissue composition of individual organs to help establish realistic pharmacokinetic relationships. Our “bottom up” in-silico PBPK model includes regional deposition data with other in-vitro data and considers physiological processes that can impact bioavailability. A robust PBPK model can help scientists to screen and optimize dosing regimens, excipients or enhancers, devices and formulations to arrive at a reliable and predictive profile for every product.
Orally inhaled and nasal drug products must comply with increasingly strict and complex regulatory requirements including U.S. FDA and EMEA. Working with Nanopharm means you have access to its team of OINDP experts who are well versed in drug device combination product regulatory requirements and can provide customers with comprehensive regulatory support. From phase appropriate drug product characterization (DPC) and testing to CMC ready data and documentation, Nanopharm’s regulatory experts are there to help. With over two decades dedicated to advancing inhaled and intranasal drug delivery products, our regulatory support helps customers to navigate today’s current regulatory landscape.
Complete OINDP Development Services
Nanopharm’s fully integrated OINDP development and analytical services have helped customers to advance their intranasal or inhaled drug product development programs. With state-of-the-art facilities, highly experienced scientists, specialized analytical techniques, and unique modeling and simulation platforms, such as our proprietary SmartTrack™ platform, Nanopharm is truly dedicated to the development of orally inhaled or intranasal drug products for its customers.