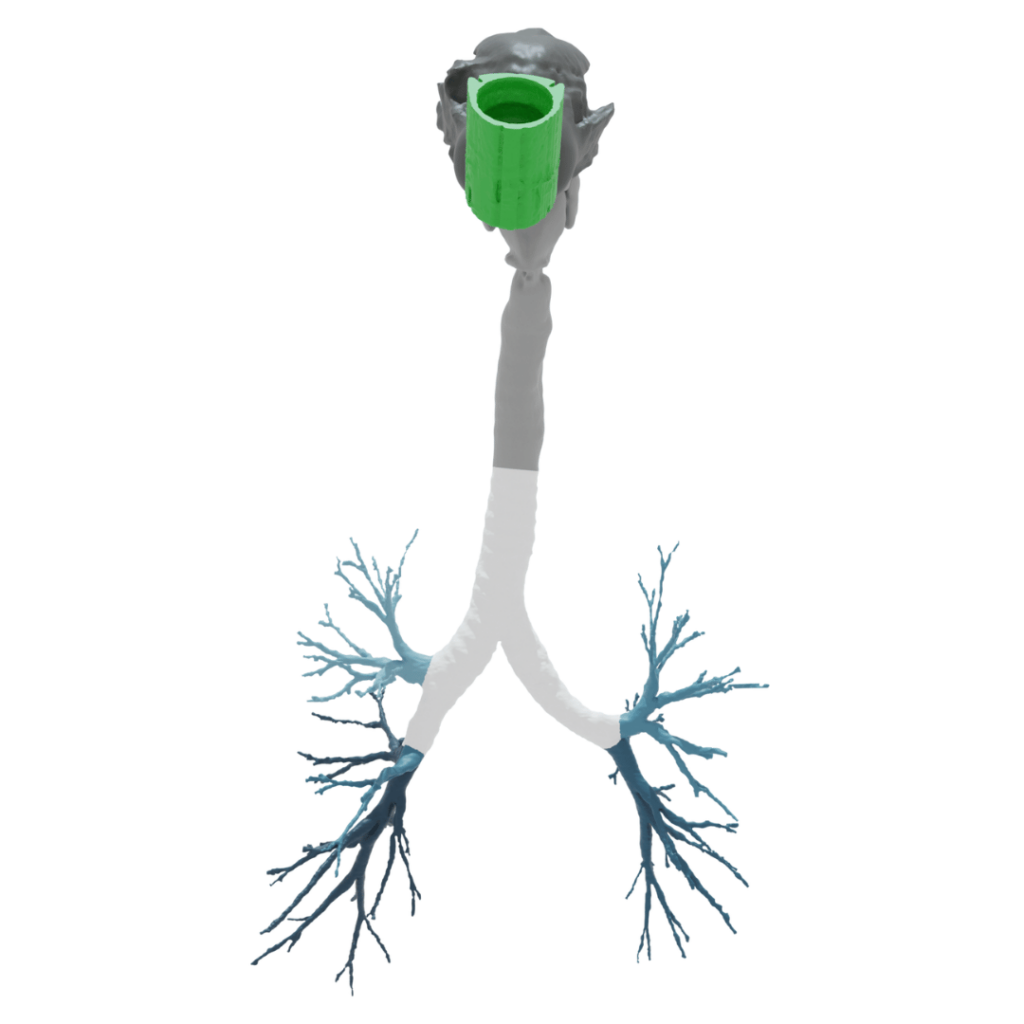
Regional Deposition Modeling
Regional deposition Modeling is critical for the development of robust nasal or inhaled drug products. A good regional deposition model can help to predict the quantity of drug deposited in parts of the lung or nasal cavity that can be absorbed into the bloodstream. This information can be used help to guide formulation, device selection, and human factor aspects of the drug’s development for optimized dosing. Nanopharm utilizes a range of advanced in-silico techniques, including computational fluid dynamics (CFD), in combination with other in-vitro and in-vivo data to develop representative regional deposition models for today’s complex OINDP products.
Nanopharm In-Silico Regional Deposition Modeling
Nanopharm’s in-silico regional deposition Modeling draws on a range of in-vivo and in-vitro data to create in-silico models that can reliably predict the quantity of drug deposited in each region of the target area. As Nanopharm’s scientists are dedicated to the development of OINDPs, their focus is on developing and improving specialized scientific tools and approaches that can accelerate and derisk the process.
Nanopharm’s regional deposition Modeling helps to define optimal devices and formulations that can deliver the drug product to the selected target region in the correct quantity.
Nanopharm uses regional deposition Modeling that include HRCT scans and Functional Respiratory Imaging (FRI) to generate disease specific data using computational fluid dynamics (CFD) simulations in collaboration with our partner FLUIDDA.
Nanopharm’s proprietary Nasal Cast system, Aeronose™, helps to visualize and quantify the amount of the drug deposited in various regions of the nasal cavity.
Regional Deposition Modeling Benefits
Regional Deposition Modeling provides information that is critical to determining the optimal device and formulation approach that will effectively deliver the desired amount of active pharmaceutical ingredient (API) to the selected target region. Depending on the formulation type, different properties can have an impact on drug deposition. For inhaled products, the device, the API properties, the way the patient uses the device and the patient’s lung anatomy all impact the regional deposition. For liquid nasal sprays, both device orientation and formulation viscosity can have a major impact on regional deposition patterns. Similarly, nasal powders, physicochemical properties such as particle size and flow characteristics can have an impact of deposition and must be accounted for in the in-silico models. Having representative in-silico regional deposition models based on a mixture of in-vitro and in-vivo data can help to accelerate product development for OINDPs and reduce costly rework at later stages. All of these data sources are incorporated into Nanopharm’s the SmartTrack™ platform to predict local drug bioavailability.
Computational Fluid Dynamics for Regional Deposition Modeling
Nanopharm’s in-silico Modeling of regional deposition is built on real-patient nasal geometries generated from High Resolution Computerized Tomography (HRCT) scans. These scans are analyzed by specialist scientists with functional respiratory imaging techniques (FRI). The HRCT scans are available by specific disease classes which can impact regional deposition results. The corresponding geometries are used as boundary conditions for flow simulations using computational fluid dynamics (CFD) platforms. Nanopharm’s OINDP scientists also assess the impact of the device on nose geometry for the evaluation of a range of human factors that can also impact drug deposition. The developed regional deposition model’s data sets are then clinically validated using in-vivo gamma scintigraphy or single photon emission computed tomography (SPECT) imposed on Computerized Tomography (CT) scans, which can more accurately quantitate deposition in targeted areas. Nanopharm uses in-vitro input data characterizing particles, spray velocity, spray patterns, and dosing to screen devices and formulations. Nanopharm’s Regional Deposition Models are designed to act as a digital clinical study that considers the patient-to-patient variability seen within subject segments.
Aeronose™ Nasal Cast In Vitro Modeling
Nanopharm uses a proprietary Nasal Cast system called Aeronose™ to visualize and accurately measure the amount of a drug or therapeutic agent that has been delivered and settled in a specific region of the nasal cavity. Quantitative deposition ensures that the desired amount of the drug reaches its target site, which is crucial for therapeutic efficacy, and is affected by both the device and the formulation properties.
Aeronose™ is a 3D in-vitro representation of the nasal cavity that can be split into separate regions for deposition quantitation. The Nasal Cast model provides in-vitro deposition data that can be incorporated into Nanopharm’s in-silico Regional Deposition models, providing a clearer picture of drug deposition in the nasal cavity relative to other model parameters.
Nanopharm Regional Deposition Modeling Specialists for Inhalation Products
Nanopharm OINDP drug development specialists utilize Regional Deposition Modeling to support the efficient and robust development of drug products as part of their comprehensive in-silico simulation capabilities.