Analytical Method Development & Validation
Being able to accurately perform qualitative and quantitative analysis using appropriate analytical test methods is complicated for inhaled and nasal drug products. Nanopharm has the ability to develop and validate the specialized analytical test methods required to characterize OINDP active pharmaceutical ingredients (APIs), drug formulations, as well as the complete drug-device combination products. We develop the test methods you need to understand your OINDP, with phase appropriate qualification and validation. Our cGMP analytical laboratory allows us to carry on performing release and stability testing as your inhaled or nasal product advances through clinical trials to market.
OINDP Analytical Development & Validation
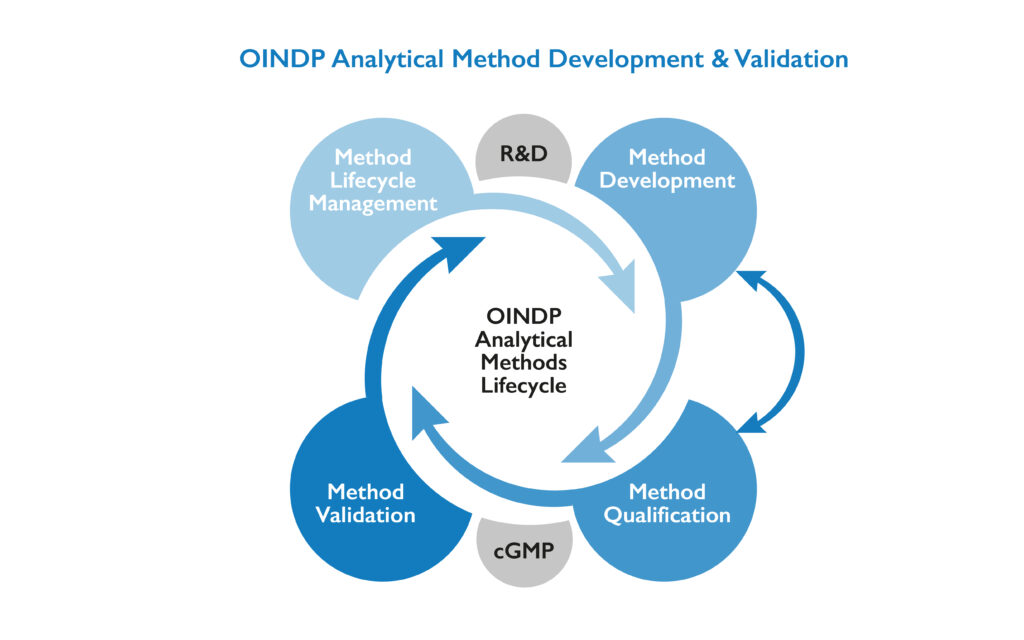
Nanopharm’s analytical scientists bring extensive knowledge in developing and validating analytical test methods for specialized inhaled and nasal drug products to every customer OINDP project. We use phase appropriate test method qualifications and validations, whether it’s in the clinic or commercial stages, to ensure our test methods work with reliability. Our experienced scientists can take existing analytical methods and fine tune them for specific drug or device applications or start from scratch and develop entirely new test methods for innovative new molecules or products. Our objective is always to develop, validate and utilize analytical test methods for OINDPs that meet the customer’s needs.
Analytical test methods for drug substances are crucial for developing Oral Inhalation and Nasal Drug Product (OINDP) formulations, applicable to both large and small molecules.
Nanopharm develops and validates OINDP analytical test methods designed to mitigate interference caused by excipients or other formulation constituents, ensuring the reliability of the results.
Drug-device combinations add an additional layer of complexity to analytical testing for inhaled or nasal drug products. Nanopharm brings specialized knowledge to develop and validate cGMP test methods for virtually any OINDP drug delivery device and wide range of formulations.
OINDP Drug Substance Analytical Test Methods
Analytical development always starts with the drug substance or API. Having a clear understanding of your API, whether it’s a complex biologic or a simple small molecule, sometimes requires the development of modified or new test methods. Analytical test methods are crucial to identifying or quantifying related substances, or degradation impurities which can impact the drug product development process at later stages. Drug substance methods can serve as the foundation for some future formulation or drug product analytical test methods including drug assays. Once our drug substance methods are developed and documented they undergo phase appropriate analytical method qualification and validation in our cGMP laboratory to ensure they are providing product specific results that can be used for data driven decision making.
OINDP Formulation Analytical Test Methods
Nanopharm has the capabilities to create new or modified analytical test methods for OINDP formulations. Even established methods will require optimization to ensure assay results avoid interference caused by excipients or components of the formulation itself. Nanopharm uses advanced analytical techniques such as dynamic light scattering to assess the stability profile of sensitive protein or peptide molecules in a variety of formulation types. Nanopharm has the capabilities to develop the analytical methods needed to characterize formulations with phase appropriate validations.
Drug-Device Combination Product Analytical Test Methods
OINDPs add an additional layer of analytical complexity as their chosen drug delivery device can have a great impact on the drug substance and formulation. Required analytical testing for drug-device combination products can include methods such as spray characterization or plume geometry analysis in order to demonstrate the the drug product is being delivered to specifications. Nanopharm brings extensive knowledge of Aptar Pharma’s OINDP devices and can develop fully optimized methods forsuch combination products. We can also work with virtually any manufacturer’s drug delivery systems and can develop unique analytical methods for novel devices where no prior test method exists. Nanopharm has the ability to create analytical test methods that characterize a broad range of drug delivery system types, and their associated products, including metered dose inhalers (MDIs), dry powder inhalers (DPIs) or nasal spray devices.
Analytical Method Development and Validation for OINDP
Nanopharm has built extensive and specialized analytical capabilities that can develop and validate, to phase appropriate standards, the analytical methods that matter for orally inhaled and nasal drug products (OINDPs). Whatever the stage of development, we can develop drug substance, formulation and drug product analytical testing methods that will ensure you can develop or release your OINDP to the clinic and market with confidence.