Nanopharm & FLUIDDA Interviews on SmartTrack: In Vitro-In Silico Approaches for Demonstrating Bioequivalence for OINDP

To support generic drug companies in obtaining a market license for their orally inhaled drug products (OIDPs), Nanopharm and FLUIDDA entered into an exclusive collaboration to combine Nanopharm’s SmartTrack™ with FLUIDDA’s Functional Respiratory Imaging technology to create a unique in-vitro in-silico platform. This allows pharma companies to shorten their clinical pathways – and potentially avoid […]
A Holistic Demonstration of SmartTrack™ and New Ways of Collaborating Combine for a Successful FDA Workshop
Nasal spray innovation with micellar delivery of Cyclosporine A holds promise in combating SARS-CoV-2 infections
Cyclosporine A micellar nasal spray characterization and antiviral action against SARS-CoV-2
Nasal spray innovation with micellar delivery of Cyclosporine A holds promise in combating SARS-CoV-2 infections
Intranasal Drug Delivery: Unravelling the Nanoparticle Formulation Puzzle
Navigate the complexities of formulating nanoparticles for intranasal drug delivery with expert insights
Dual Excipient Integration in Dry Powder Inhaler Products
Nanopharm explore the effect of dual excipients on DPI products, boosting performance and shaping formulation in the dry powder inhaler landscape.
Optimising batch selection to increase success of PK studies for bioequivalence
Uncover Nanopharm’s SmartTrack approach for successful Pharmacokinetic and Bioequivalence studies in OINDP, using PBPK modelling.
Physiologically-based Pharmacokinetic Models (PBPK) for OINDPs

Aptar Pharma discusses Physiologically based PK models for OINDPs.
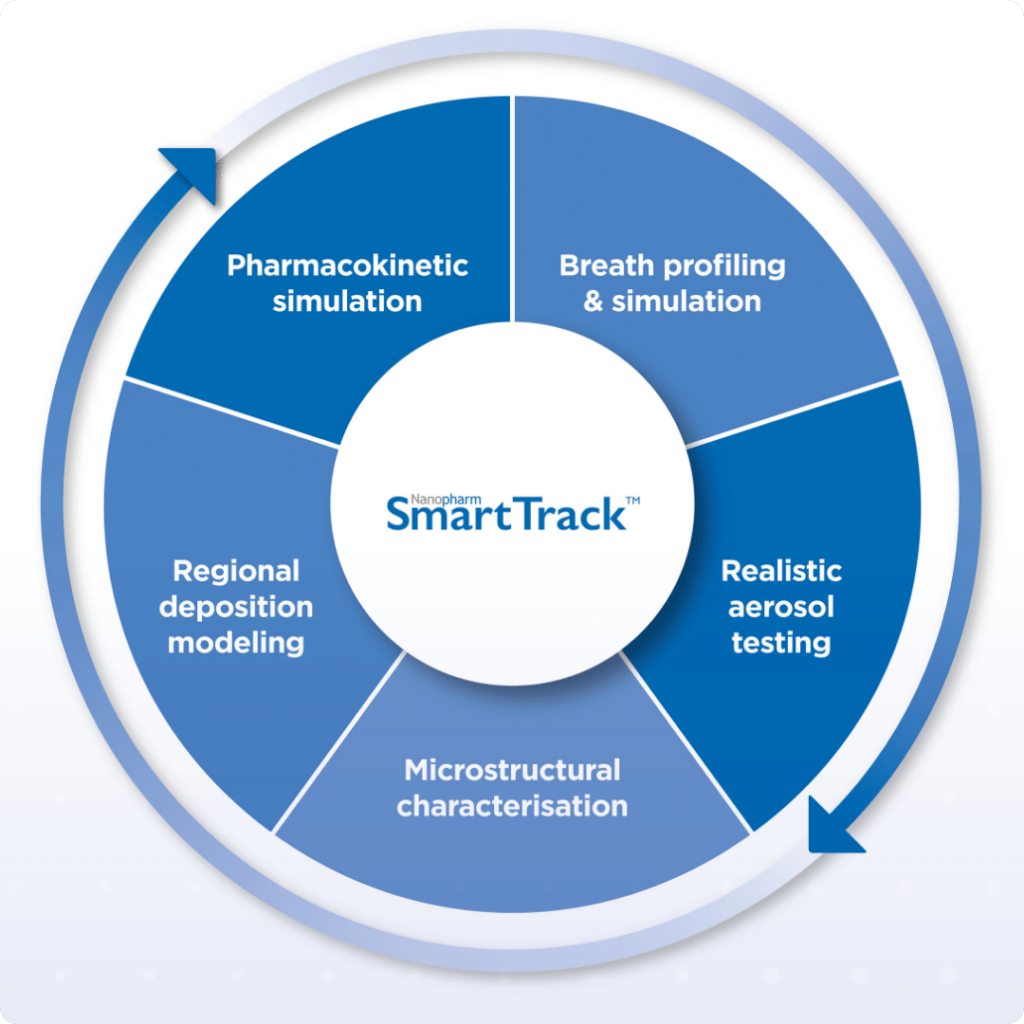
Nanopharm’s SmartTrack: Accelerating OINDP Respiratory Drug Delivery

Revolutionize your OINDP respiratory drug delivery with Nanopharm’s SmartTrack. Accelerate development and mitigate risks through expert formulation and integrated solutions. Your pathway to success starts here.
Nanopharm’s Role in OINDP Bioequivalence & Pharmacokinetics
Explore Nanopharm’s groundbreaking methods in OINDP bioequivalence and pharmacokinetics. Learn how in vitro and in vivo studies are revolutionizing drug development and solubility.
Nasal Drug Development, Trends and Challenges with Nasal Formulations

Nanopharm explores the current trends and technical challenges in nasal drug product development, focusing on formulation.
