Accelerating OINDP Development with Nanopharm

Discover how Nanopharm, supports OINDP development from preclinical to CGMP with SmartTrack™, in silico modeling, and regulatory expertise.
Advancing Alternative Bioequivalence for OINDPs

Nanopharm & Fluidda explore FDA’s progress in alternative bioequivalence for OINDPs, Model Master Files (MMF), and future regulatory pathways.
In Silico Bioequivalence Beyond Generic Drug Development

Nanopharm & Fluidda discuss FDA’s in silico bioequivalence methods for alternative pathways beyond generics in inhalation drug development.
FDA’s Evolving Stance on In-Silico Studies in Bioequivalence Testing

Discover how the FDA’s evolving view on in-silico methods, with insights from Nanopharm and FLUIDDA, impacts bioequivalence and generic drugs.
FDA New Inhaled Product Guidelines: Bioequivalence & PSGs

Explore Nanopharm & Fluidda discuss FDA’s revised inhaled product guidelines (PSGs) and alternative bioequivalence pathways for generics.
Nanopharm & Fluidda Discuss FDA’s New Inhaled Product Guidelines

Explore how alternative bioequivalence methods, including computational fluid dynamics and in silico modeling, are shaping the future of generic drug approval.
Nanopharm Announces Exclusive Collaboration With Fluidda To Accelerate Regulatory Pathway For OINDP Using SmartTrack

Crystal Lake, Illinois, September 22, 2022 – Nanopharm, an Aptar Pharma company and leader in contract research and development services for orally inhaled and nasal drug products (OINDPs), today announced an exclusive collaboration with Fluidda, a leader in the field of Functional Respiratory Imaging. The companies will leverage their respective proprietary technology platforms to help accelerate U.S. Food & Drug Administration (FDA) approvals for orally inhaled generic products (OIDPs) via the alternative bioequivalence pathway. Nanopharm was acquired by Aptar (NYSE: ATR) in 2019, as part of the company’s strategy to expand its services offerings and partner with pharmaceutical companies earlier in the drug development process.
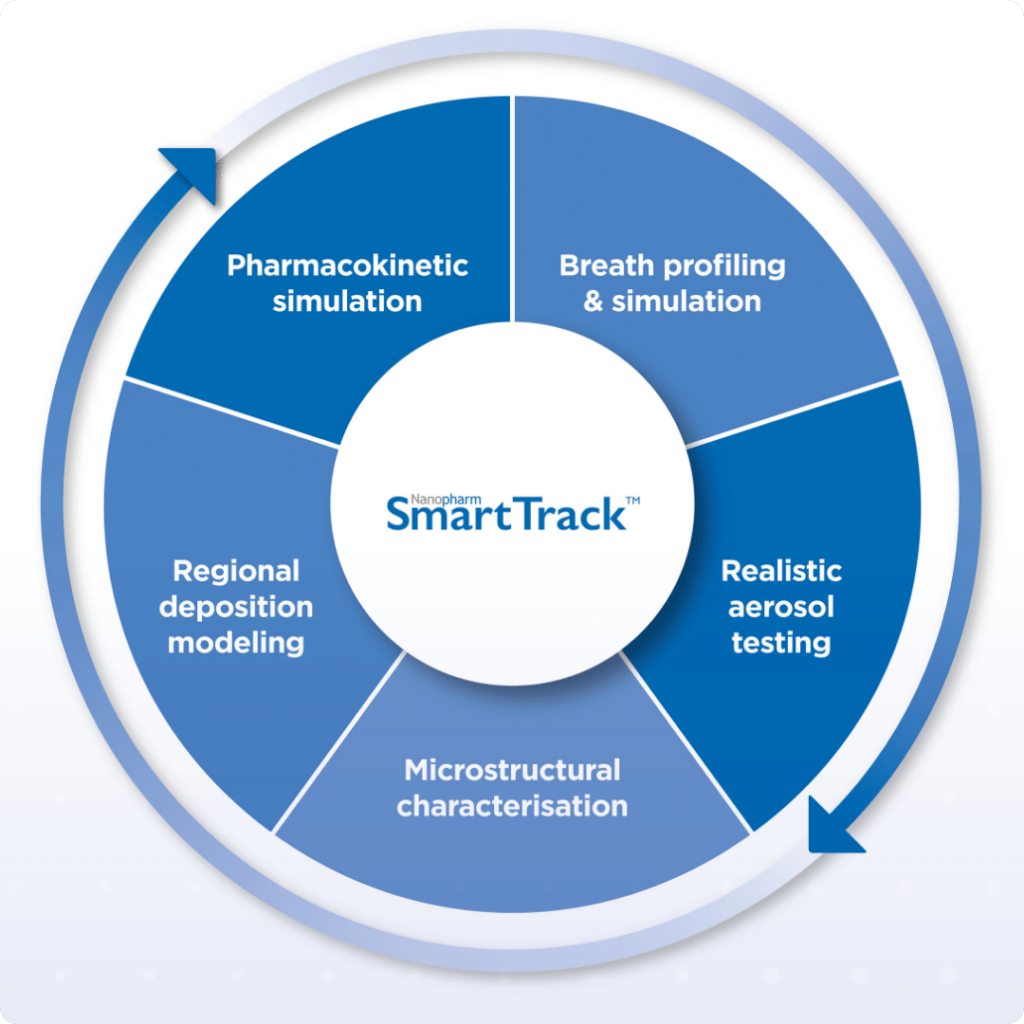
Nanopharm & FLUIDDA Interviews on SmartTrack: In Vitro-In Silico Approaches for Demonstrating Bioequivalence for OINDP

To support generic drug companies in obtaining a market license for their orally inhaled drug products (OIDPs), Nanopharm and FLUIDDA entered into an exclusive collaboration to combine Nanopharm’s SmartTrack™ with FLUIDDA’s Functional Respiratory Imaging technology to create a unique in-vitro in-silico platform. This allows pharma companies to shorten their clinical pathways – and potentially avoid […]
A Holistic Demonstration of SmartTrack™ and New Ways of Collaborating Combine for a Successful FDA Workshop
Nasal spray innovation with micellar delivery of Cyclosporine A holds promise in combating SARS-CoV-2 infections
Cyclosporine A micellar nasal spray characterization and antiviral action against SARS-CoV-2
Nasal spray innovation with micellar delivery of Cyclosporine A holds promise in combating SARS-CoV-2 infections
