OINDP Expertise
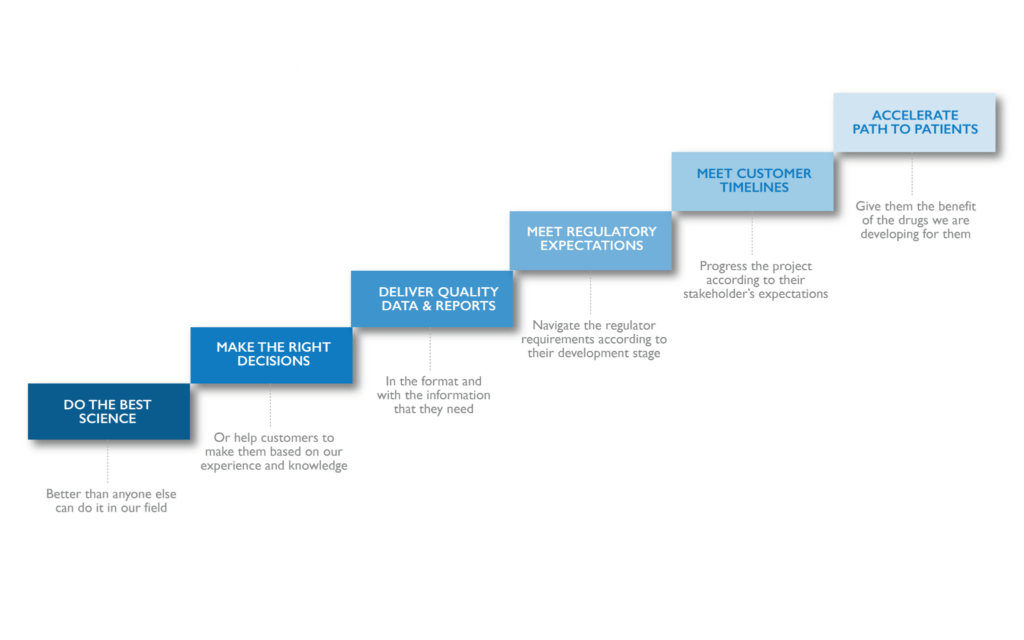
Nanopharm has built its comprehensive intranasal and inhaled drug development capabilities with industry leading OINDP expertise through decades of scientific dedication. Whether it’s for nasal sprays or powders, or for a range of inhalation technologies, including Dry Powder Inhalers (DPIs), Pressurized Metered Dose Inhalers (pMDIs), Nebulizers, and Soft Mist Inhalers (SMIs), Nanopharm has the specialized technologies and knowledge needed to advance OINDP development. For biologics or small molecules, Nanopharm develops products that can meet customer requirements and regulatory needs. We have the ability to provide bioequivalence via traditional well-defined regulatory pathways as well as new alternative non-clinical pathways that you can’t find elsewhere. We work closely with regulators so that our leading edge in-vitro and in-silico platforms can meet their requirements, negating the need for costly and time-consuming human studies. The future of OINDP development starts with Nanopharm.
Nanopharm OINDP Development Expertise
OINDPs can provide a number of important benefits over other conventional dosage forms. As inhaled or nasal drug delivery routes are more complex, they require specialized knowledge to develop these dosage forms successfully. That’s where Nanopharm comes in. We apply decades of experience gained through developing leading OINDPs for a wide range of customers, using our proprietary technologies and approaches to arrive at scalable, robust inhaled and nasal drug products. OINDPs can provide a convenient, portable, differentiated, and reliable drug delivery format for a variety of today’s drug products. Let Nanopharm derisk and accelerate your next OINDP project.
Nanopharm offers specialized intranasal product development services for Nasal Powder and Liquid Nasal Spray products. Using leading-edge technologies, combined with decades of experience Nanopharm can develop nasal products that meet today’s strictest requirements.
Nanopharm’s scientists can develop inhaled drug products such Dry Powder Inhalers (DPIs), Pressurized Metered Dose Inhalers (pMDIs), Nebulizers, and Soft Mist Inhalers (SMIs) using specialized technologies and decades of experience to arrive at robust inhaled product formulations.
With inhaled biologic drug products increasingly in demand, Nanopharm has the capabilities and technologies to deliver stable inhaled biologic product formulations.
Whether its new generic product development or the repositioning of existing drug products into nasal or inhaled drug delivery formats, Nanopharm has the unique and specialized capabilities needed to accelerate and derisk the process. Repositioning products for enhanced sustainability or market differentiation are well within Nanopharm’s comprehensive service offering. Nanopharm uniquely has the capability to demonstrate bioequivalence of generic drug products through well-established regulatory pathways or alternative non-clinical pathways.
Nanopharm’s OINDP specialist scientists employ several leading-edge technologies, combined with decades of experience to develop a range of products including liquid and powder nasal sprays. With unique in-vitro and in-silico modeling platforms, Nanopharm can accelerate and derisk nasal product development, whether by traditional regulatory pathways or using advanced alternative pathways to bioequivalence. Both biologics and small molecule products can enjoy the advantages nasal drug delivery, which include portablility, non-invasive administration and they are easy to administer. Nanopharm also uses specialized analytical tools to support rapid and robust nasal product development including Morphologically Directed Raman Spectroscopy (MDRS), dissolution testing and our proprietary Aeronose Nasal CAST 3D in-vitro nasal cavity model. With comprehensive particle engineering options, we can develop nasal powder formulations that may also provide added advantages in product stability and high-dose loading. Let Nanopharm advance your next nasal drug delivery product to the clinic and ultimately to the patients that need them.
Nanopharm is focused on the advancement of inhaled drug delivery science. Our established respiratory scientists can develop inhaled drug products such Dry Powder Inhalers (DPIs), Pressurized Metered Dose Inhalers (pMDIs), Nebulizers, and Soft Mist Inhalers (SMIs) that benefit end users. Inhalation is a complicated drug delivery pathway, but can reliably provide direct drug delivery to targetted areas of the lungs for both local and systemic applications. Our DPI development services can include sophisticated material characterization, process development, carrier selection, particle engineering, and aerodynamic performance testing. With access to Aptar Pharma’s widely used metering valves, Nanopharm’s pressurized metered-dose inhaler (pMDI) product development scientists analyze the drug delivery system, formulation and breath profiles to arrive at highly optimized pMDIs. Nebulizers and soft mist inhalers (SMIs) can also provide a viable route to deliver large volumes of low concentration formulation to the lungs for chronic respiratory conditions. No matter your inhalation drug delivery approach, Nanopharm has the scientists with the experience and the advanced technologies required to produce manufacturable and scalable inhaled product solutions for customers.
Nanopharm has the capabilities to develop biologics for inhaled drug delivery applications. Our scientists understand the importance of maintaining the delicate structure and orientation of biologic molecules when designing formulations that work with the selected drug delivery inhalation technology. Biologics can be soluble in aqueous media but must maintain their structure in solution. Stability can prove a real challenge for biologics, especially at the relatively high concentrations required for inhaled or nasal drug product formulations. Our scientists have the knowledge and tools that are required to develop biologic OINDPs – whether they are small peptides or large antibodies or even nucleic acids encapsulated in nanoparticles. Biologic OINDPs can potentially even reduce the side effect profile and avoid the systemic side effects sometimes seen with injectables. Let Nanopharm’s experienced scientists unlock all the advantages of inhaled or nasal drug delivery for your next OINDP biologic product.
Repositioning an existing drug to an inhaled or nasal drug product can be complex but can also provide marketing differentiation because of its non-invasive and convenient drug delivery properties. Nanopharm can reformulate existing drug products, develop pMDI products that use lower Global Warming Potential (GWP) propellant options for improved sustainability, and perform in-vitro bioequivalence (IVBE) studies that can demonstrate alternative bioequivalence for orally inhaled or nasal drug products. New OINDP generic products also require that they can clearly demonstrate bioequivalence to reference products in compliance with current regulatory requirements. Nanopharm’s SmartTrack™ platform can provide a non-clinical alternative pathway to bioequivalence for inhaled drug products without having to conduct time consuming and costly human clinical studies. With our specialized testing and development expertise we can quickly and reliably develop robust OINDP drug products using the latest advances in inhaled and nasal drug product development.
Complete OINDP Development Expertise
Nanopharm’s fully integrated OINDP development and analytical services have helped many customers to advance their intranasal or inhaled drug products to market. With state-of-the-art facilities, highly experienced scientists, specialized analytical techniques, and unique modeling and simulation platforms such as our proprietary SmartTrack™ system, Nanopharm is an OPNDP specialist fully dedicated to the development of orally inhaled or intranasal drug products.