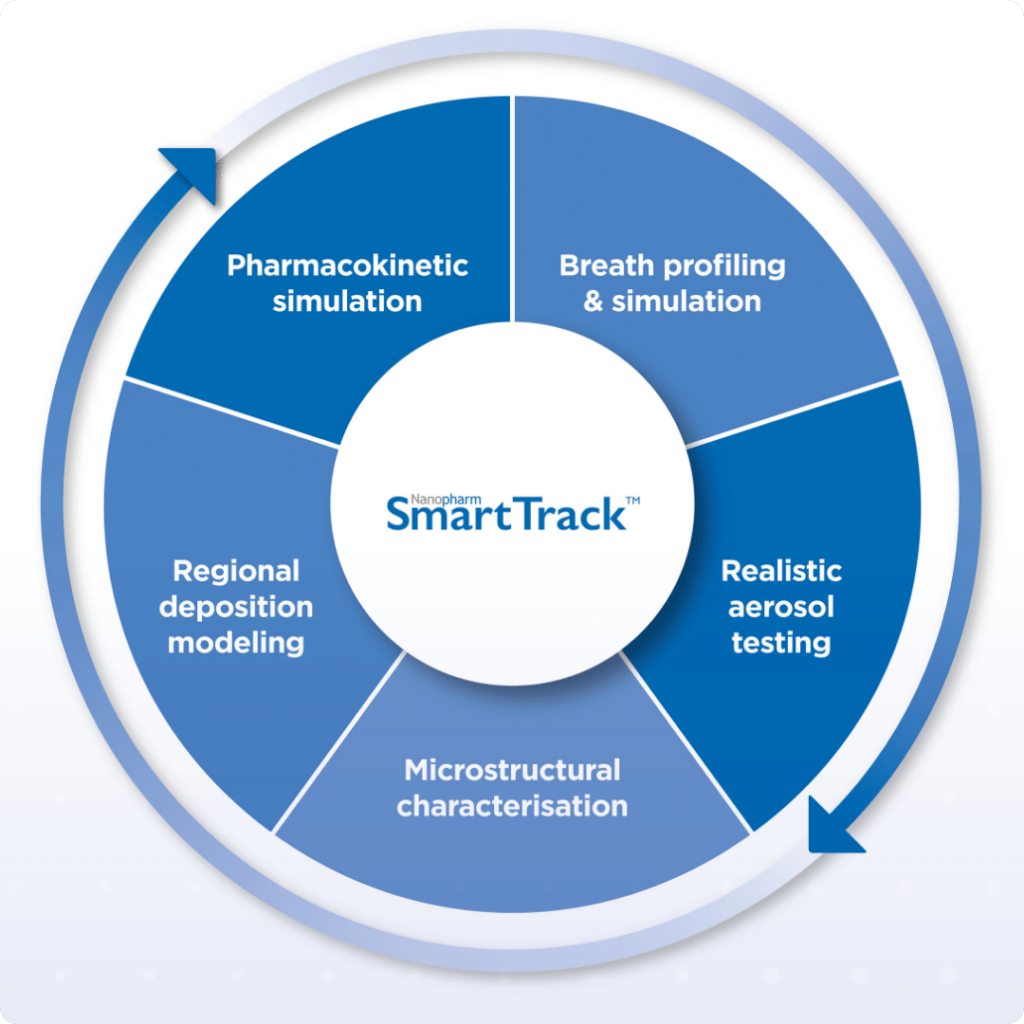
Dr. Jag Shur, Vice President, Science & Technology at Nanopharm, and Dr. Will Ganley, Head of Computational Pharmaceutics, recently discussed SmartTrack™ and Nanopharm’s extended capabilities in support of alternative bioequivalence at a workshop which was held jointly between the FDA and the Center for Research on Complex Generics (CRCG) at the University of Maryland, Baltimore and the University of Michigan.
Jag Shur’s presentation focused on realistic in vitro tools. Our partner Jan De Backer from FLUIDDA spoke on their in silico CFD models for studying regional deposition in patient-specific models.
Will Ganley, whose presentation on how in silico patient-specific PBPK models form part of a platform as an alternative to CCEP BE studies for inhaled generics, said this about the event itself: “The event was unique in that of the 85 in-person attendees a large proportion of them were from the FDA. This allowed us to have conversations and ask questions during the sessions and over lunch or coffee, which I had never experienced before. The workshop opened with a statement that only 4/33 of currently approved inhaled medicines in the US have approved generics which was motivating.”
Will said, “The talks covered a wide range of topics from the shortcomings of current bioequivalence approaches to novel or alternative in silico, in vitro and in vivo studies that could help us better demonstrate bioequivalence for orally inhaled products. There was a lot of healthy discussion, especially during the small group discussions and panel sessions. There were a lot of differences of opinion on the best way to bring the industry’s expertise together to solve this problem, but it was clear that the basic research is mainly done. We have already established the tools we need to make this happen. The final challenge is figuring out how to bring them all together for each of the remaining products that don’t have approved generics.”
“Overall, the event was excellent. We covered a lot of ground, and the attendees were keen to share experience and opinions. My perspective has shifted on a few things. Still, I am confident that Nanopharm’s approach of bringing relevant in vitro studies and in silico methods together ticks all the boxes.”
More information about the event can still be found here.

Related Posts

Rethinking bioequivalence for nasal suspensions: Adopting an in-vitropathway with confidence
Discover how MDRS provides a regulatory-accepted alternative to clinical endpoint studies for nasal suspension bioequivalence. Learn to de-risk your ANDA pathway today.

Automating pMDI Actuation for Consistent In Vitro Bioequivalence Testing
Discover how automated pMDI actuation improves consistency and reduces analyst variability in in vitro bioequivalence testing, supporting realistic APSD analysis and FDA expectations.

Targeting accelerated progress at the IPAC-RS Nasal Innovation Forum 2025
Explore how Nanopharm presented strategies for accelerating patient-centric nasal product development at the IPAC-RS Nasal Innovation Forum 2025. Dive into key insights on regulatory science, advanced bioequivalence methods, and targeted drug delivery.